
During COVID-19 pandemic, patent case filings are slowing… and surging
To better understand how the pandemic has impacted patent litigation filings throughout the U.S., we compared 2019 & 2020 new patent cases.

To better understand how the pandemic has impacted patent litigation filings throughout the U.S., we compared 2019 & 2020 new patent cases.

On June 30, major advertisers announced a boycott of advertising through Facebook. Will it work?

The biggest technology companies have found themselves entangled in the latest chapter of China and Hong Kong’s long, complicated political history.

To date, social media companies and other online technology platforms have operated virtually unregulated. Big changes may be coming.

There is growing unease about the use of drones and other high-tech surveillance techniques to monitor peaceful protests.

The US Justice Department appears poised to bring an antitrust case against Google. If it does, this could be the biggest antitrust case in United States history.

For many students, families, and businesses, Zoom is an ‘essential service.’ But data security issues with the platform are drawing unwanted attention, criticism, and even lawsuits.

Health, safety, and legal liability are hot-button issues for employers as the U.S. economy positions to reopen. Many businesses fear the potential for legal minefields surfacing in a post-Covid-19 world, and with good reason – the minefields exist.

In a piecemeal regulatory environment, consumers will bear the brunt of the responsibility for vetting any app they choose to give access to their medical information. How that information is protected will be the province of third parties – until legislated otherwise.
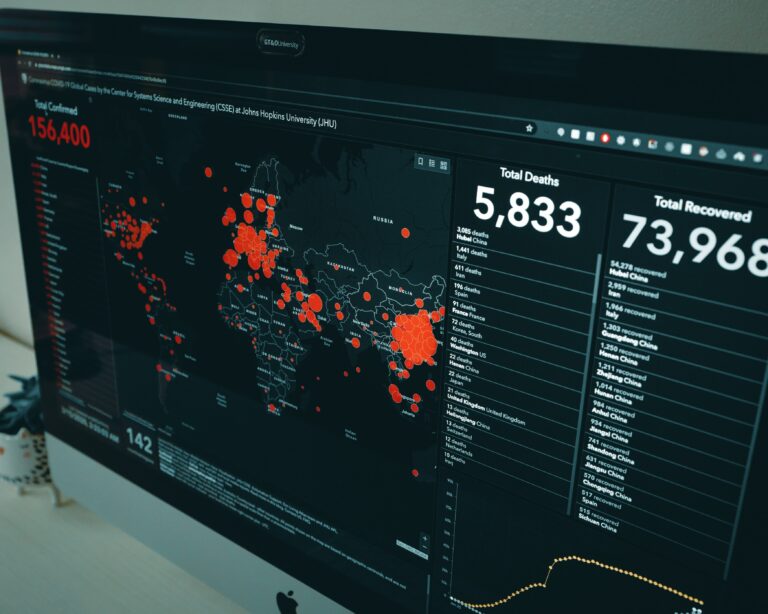
Artificial intelligence is not a silver bullet, its ability to efficiently and accurately crunch numbers is aiding the containment and treatment effort for COVID. Lessons learned now will help future outbreaks. No longer the realm of sci-fi, AI is in the fight against the pandemic.